How to Read Stats in a Research Article
The tables in this department present the research findings that bulldoze many recommendations and standards of practice related to breast cancer.
Enquiry tables are useful for presenting information. They show a lot of information in a elementary format, just they tin exist hard to understand if y'all don't piece of work with them every mean solar day.
Hither, nosotros draw some bones concepts that may help yous read and understand research tables. The sample table below gives examples.
The numbered table items are described beneath. You will meet many of these items in all of the tables.
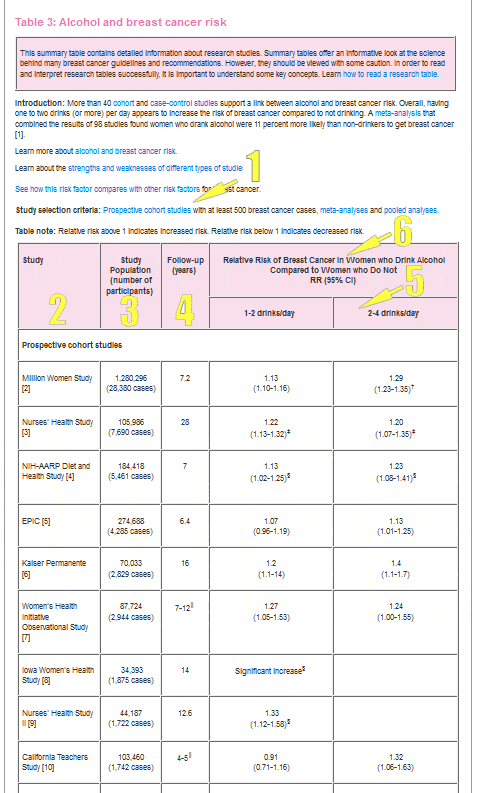

Option criteria
Studies vary in how well they help respond scientific questions. When reviewing the research on a topic, it's important to recognize "good" studies. Skilful studies are well-designed.
Most scientific reviews set standards for the studies they include. These standards are called "choice criteria" and are listed for each table in this section.
Types of studies
The types of studies (for example, randomized controlled trial, prospective cohort, case-control) included in each table are listed in the selection criteria.
Learn about the strengths and weaknesses of unlike types of research studies.
Study size
Selection criteria for well-nigh tables include the minimum number of cases of chest cancer or participants for the studies in the table.
When all else is equal, a larger number of people in a study means the study is ameliorate able to respond inquiry questions. While there are large, poorly-designed studies, in general, large studies are meliorate than small ones.
Larger studies accept more statistical power. This ways the results from large studies are less likely to be due to chance than results from small studies.
Learn more than most report size and statistical power.
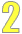
The studies
The starting time cavalcade (from the left) lists either the name of the study or the name of the first author of the published commodity.
Below each table, there's a reference list so you can find the original published articles.
Sometimes, a table volition written report the results of but i analysis. This can occur for a few reasons. Either there's only one report that meets the option criteria or there's a report that combines information from many studies into one big assay.
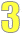
Study population
The 2nd column describes the people in each report.
- For randomized controlled trials, the study population is the total number of people who were randomized at the showtime of the study to either the treatment (or intervention) grouping or the control group.
- For prospective cohort studies, the written report population is the number of people at the start of the written report (baseline cohort).
- For instance-control studies, the report population is the number of cases and the number of controls.
In some tables, more than details on the people in the study are included.
Length of follow-upward
Randomized controlled trials and prospective cohort studies follow people forward in fourth dimension to come across who will have the outcome of interest (such as breast cancer).
For these studies, one column shows the length of follow-up time. This is the number or months or years people in the written report were followed.
Because case-command studies don't follow people forward in time, there are no information on follow-up time for these studies.
Tables that focus on cumulative gamble may as well show the length of follow-up. These tables give the length of time, or historic period range, used to compute cumulative risk (for instance, the cumulative chance of breast cancer upwards to historic period 70).
Acquire more than nearly cumulative chance.
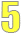
Other information
Some tables have columns with other information on the study population or the topic being studied. For case, the table Exercise and Breast Cancer Run a risk has a cavalcade with the comparisons of exercise used in the studies.
This extra information gives more than details about the studies and shows how the studies are like to (and different from) each other.
Studies on the same topic tin can differ in of import means. They may define "high" and "low" levels of a take chances factor differently. Studies may expect at outcomes among women of different ages or menopausal status.
These differences are important to keep in mind when you review the findings in a table. They may help explain differences in study findings.
Understanding the numbers
All of the data in the tables is important, but the main purpose of the tables is to present the numbers that show the risk, survival or other measures for each topic. These numbers are shown in the remaining columns of the tables.
The headings of the columns tell you what the numbers correspond. For example:
- What is the outcome of involvement? Is it chest cancer? Is information technology 5-twelvemonth survival? Is it chest cancer recurrence?
- Are groups being compared to each other? If so, what groups are being compared?
Relative risks
Nearly often, findings are reported as relative risks. A relative take chances shows how much higher, how much lower or whether there's no difference in risk for people with a certain take a chance factor compared to the risk in people without the factor.
A relative risk compares 2 absolute risks.
- The numerator (the elevation number in a fraction) is the accented run a risk among people with the take a chance factor.
- The denominator (the bottom number) is the absolute risk amid those without the risk factor.
The accented risk of those with the gene divided past the absolute risk of those without the cistron gives the relative risk.
![]()
| When relative take chances is: | This shows: |
| Greater than 1 | People with the risk factor accept a higher adventure than people without the risk factor. A relative risk of 1.v means someone with the risk cistron has a 50 percent higher take chances of breast cancer than someone without the factor. A relative risk of two.0 ways someone with the adventure cistron has twice the risk (or ii-fold the run a risk) of someone without the factor. |
| Less than ane | People with the risk cistron have a lower risk than people without the run a risk cistron. A relative risk of 0.8 means someone with the risk factor has a xx percent lower risk of breast cancer than someone without the factor. |
| i | A relative adventure of one means there'south no difference in adventure betwixt people with and without the gamble factor. |
The confidence interval around a relative risk helps show whether or non the relative hazard is statistically meaning (whether or not the finding is likely due to risk).
Learn more nigh confidence intervals.
Example of relative risk
Say a study shows women who don't exercise (inactive women) have a 25 percent increase in breast cancer hazard compared to women who practice do (active women).
This statistic is a relative risk (the relative risk is 1.25). Information technology means the inactive women were 25 percent more likely to develop chest cancer than women who exercised.
Acquire more near relative risk.
Confidence intervals
A 95 percentage confidence interval (95% CI) around a risk measure ways there's a 95 percentage gamble the "true" measure out falls inside the interval.
Because there'south random error in studies, and written report populations are but samples of much larger populations, a single study doesn't give the "ane" correct respond. There'southward always a range of likely answers. A unmarried written report gives a "all-time estimate" along with a 95 % CI of a likely range.
Well-nigh scientific studies report gamble measures, such equally relative risks, odds ratios and averages, with 95% CI.
Confidence intervals and statistical significance
For relative risks and odds ratios, a 95% CI that includes the number 1.0 means there'south no link between an exposure (such as a risk factor or a treatment) and an outcome (such as chest cancer or survival).
When this happens, the results are not statistically pregnant. This ways whatsoever link betwixt the exposure and result is likely due to hazard.
If a 95% CI does not include 1.0, the results are statistically significant. This ways there's probable a true link between an exposure and an event.
Examples of confidence intervals
A few examples from the sample table above may assistance explicate statistical significance.
Examples
A few examples from the sample tabular array may better explicate the concept of statistical significance.
The EPIC study establish a relative risk of chest cancer of 1.07, with a 95% CI of 0.96 to 1.19. In the table, you lot will encounter one.07 (0.96-1.19).
Women in the Epic report who drank 1-2 drinks per day had a 7 percent college risk of breast cancer than women who did not potable alcohol. The 95% CI of 0.96 to 1.19 includes 1.0. This means these results are not statistically significant and the increased chance of breast cancer is probable due to chance.
The Million Women's Study found a relative take a chance of breast cancer of ane.xiii with a 95% CI of 1.10 to ane.16. This is shown as i.13 (one.x-1.xvi) in the table.
Women in the Million Women's Study who drank 1-2 drinks per day had a 13 percent higher risk of breast cancer than women who did not beverage alcohol. In this case, the 95% CI of i.x to i.sixteen does not include 1.0. And so, these results are statistically significant and suggest there's likely a true link between alcohol and breast cancer.
For any topic, it's important to look at the findings as a whole. In the sample table above, about studies show a statistically significant increase in risk among women who drink booze compared to women who don't drinkable alcohol. Thus, the findings as a whole suggest alcohol increases the gamble of breast cancer.
Summary relative risks
Summary relative risks from meta-analyses
A meta-assay takes relative risks reported in unlike studies and "averages" them to come up with a single, summary measure out. Findings from a meta-analysis tin give stronger conclusions than findings from a unmarried report.
Summary relative risks from pooled analyses
A pooled assay uses data from multiple studies to requite a summary measure. It combines the data from each person in each of the studies into one large data prepare and analyses the information as if it were one big written report. A pooled analysis is well-nigh e'er better than a meta-assay.
In a meta-analysis, researchers combine the results from dissimilar studies. In a pooled analysis, researchers combine the individual data from the dissimilar studies. This usually gives more statistical ability than a meta-analyses. More statistical power ways it'due south more likely the results are not simply due to chance.
Cumulative risk
Sometimes, study findings are presented every bit a cumulative hazard (risk up to a certain historic period). This risk is often shown as a percentage.
A cumulative adventure may show the risk of breast cancer for a certain group of people up to a sure age. Say the cumulative take a chance up to age 70 for women with a risk gene is 20 pct. This means by age seventy, 20 pct of the women (or 1 in 5) with the risk factor will get breast cancer.
Lifetime risk is a cumulative risk. It shows the risk of getting chest cancer during your lifetime (or upwardly to a certain age). Women in the U.Southward. accept a 13 pct lifetime risk of getting breast cancer. This ways 1 in 8 women in the U.Due south. will get breast cancer during their lifetime.
Acquire more than almost lifetime chance.
Sensitivity and specificity
Some tables evidence report findings on the sensitivity and specificity of screening tests. These measures describe the quality of a breast cancer screening examination.
- Sensitivity shows how well the screening test shows who truly has breast cancer. A sensitivity of ninety pct means 90 pct of people tested who truly have chest cancer are correctly identified as having cancer.
- Specificity shows how well the screening test shows who truly does not take chest cancer. A specificity of 90 percent ways 90 percentage of the people who practise not have chest cancer are correctly identified every bit non having cancer.
The goals of any screening exam are:
- To correctly identify everyone who has a certain illness (100 percent sensitivity)
- To correctly identify everyone who does not have the disease (100 pct specificity)
A perfect exam would correctly identify everyone with no mistakes. There would be no:
- False negatives (when people who accept the disease are missed by the test)
- False positives (when good for you people are incorrectly shown to have the disease)
No screening test has perfect (100 percent) sensitivity and perfect (100 percent) specificity. There'due south e'er a merchandise-off betwixt the 2. When a test gains sensitivity, it loses some specificity.
Larn more about sensitivity and specificity.
Finding studies
You may want more item about a written report than is given in the summary table. To assist you find this information, the references for all the studies in a table are listed below the table.
Each reference includes the:
- Authors of the study article
- Championship of the commodity
- Year the article was published
- Title and specific result of the medical periodical where the article appeared
PubMed, the National Library of Medicine's search engine, is a proficient source for finding summaries of science and medical journal manufactures (called abstracts).
For some abstracts, PubMed likewise has links to the full text articles. Most medical journals accept websites and offering their articles either for complimentary or for a fee.
If yous alive near a academy with a medical school or public health school, you lot may be able to become to the schoolhouse's medical library to get a re-create of an commodity. Local public libraries may not behave medical journals, merely they may be able to discover a re-create of an commodity from some other source.
More data on research studies
If y'all're interested in learning more most health research, a basic epidemiology textbook may be a practiced place to start. The National Cancer Institute also has information on epidemiology studies and randomized controlled trials.
Updated 12/16/20
williamsgleir1983.blogspot.com
Source: https://www.komen.org/breast-cancer/facts-statistics/research-studies/how-to-read-a-research-table/
Enregistrer un commentaire for "How to Read Stats in a Research Article"